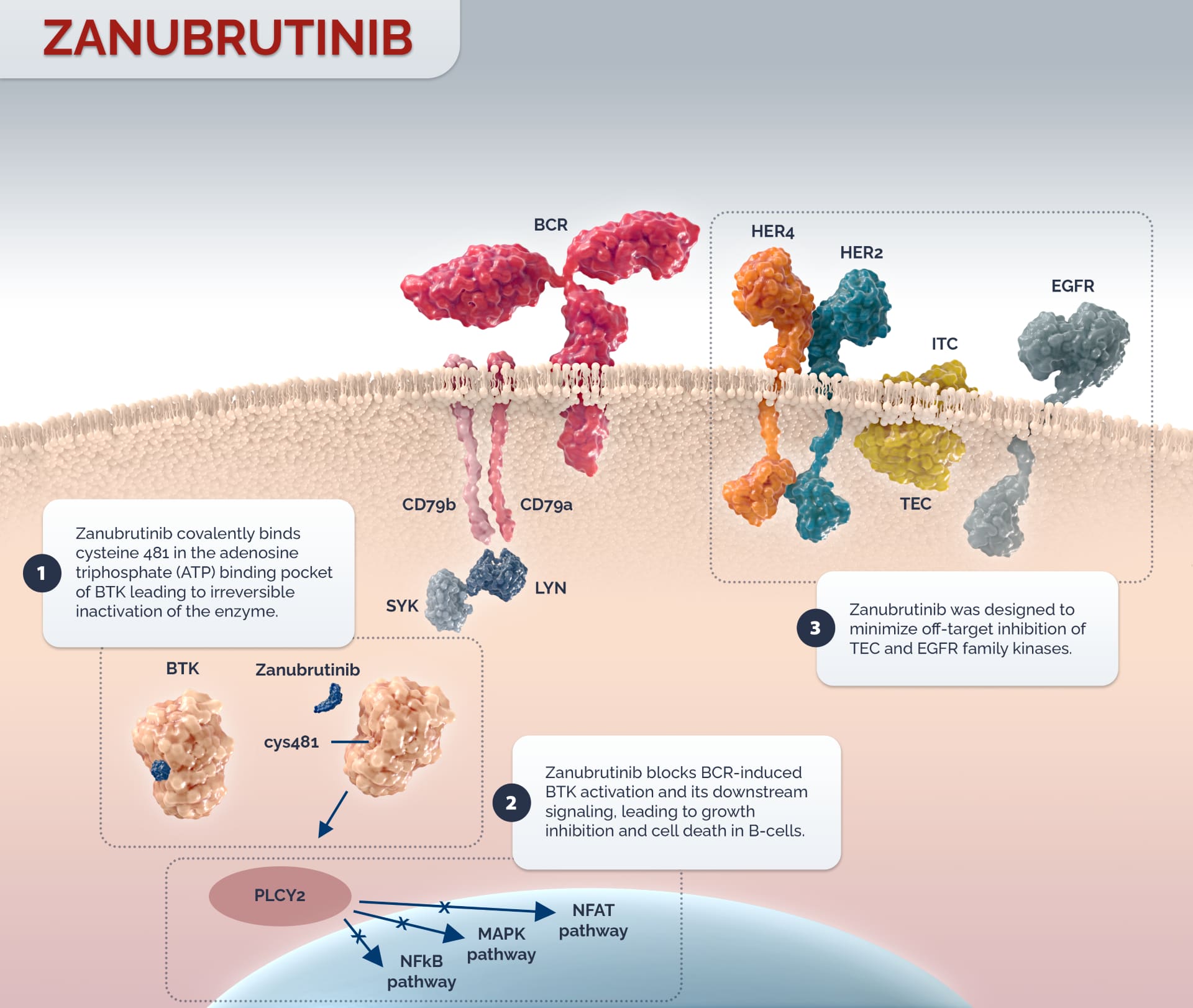
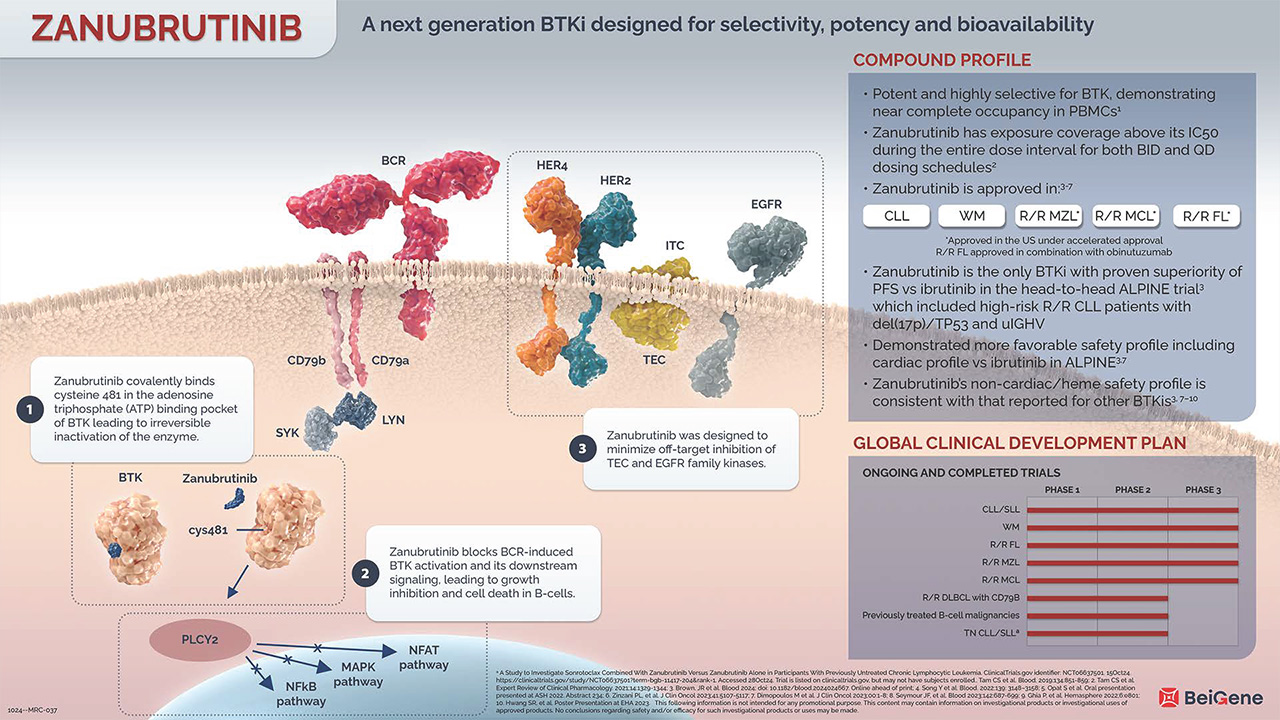
Zanubrutinib is a potent and highly selective small molecule inhibitor of Bruton’s tyrosine kinase (BTK), with demonstrated near complete BTK occupancy in peripheral blood mononuclear cells (PBMCs).1 It has exposure coverage above its IC50 during the entire dose interval for both BID and QD dosing schedules.2
Zanubrutinib is currently being evaluated in over 35 trials across 29 countries. In head-to-head clinical trials, zanubrutinib demonstrated a more favorable safety profile with fewer cardiovascular toxicities than ibrutinib. Zanubrutinib’s non-cardiac safety profile is consistent with that reported for other BTKis.3,4 Zanubrutinib is the only BTKi with proven superiority of PFS vs ibrutinib in the ALPINE trial, which included high-risk relapsed/refractory chronic lymphocytic leukemia (CLL) patients with del(17p)/TP53 and uIGHV.3
Zanubrutinib combination studies include sonrotoclax (BCL2 inhibitor), tislelizumab (anti-PD-1 mAb), BGB-10188 (PI3Kδ inhibitor), obinutuzumab (anti-CD-20 mAb), rituximab (anti-CD-20 mAb), lenalidomide + rituximab, and venetoclax (BCL2 inhibitor).
References
- Tam, C. S. et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019;134:851–859.
- Tam, C. S., Ou, Y. C., Trotman, J. & Opat, S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev. Clin. Pharmacol 2021;14(11):1329-1344.
- Brown, J. R. et al. Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: Final comparative analysis of ALPINE. Blood 2024; blood.2024024667.
- Dimopoulos, M. A, et al. Zanubrutinib versus ibrutinib in symptomatic Waldenström macroglobulinemia: Final analysis from the randomized phase III ASPEN study. J. Clin. Oncol. 2023;41(33):5099-5106.