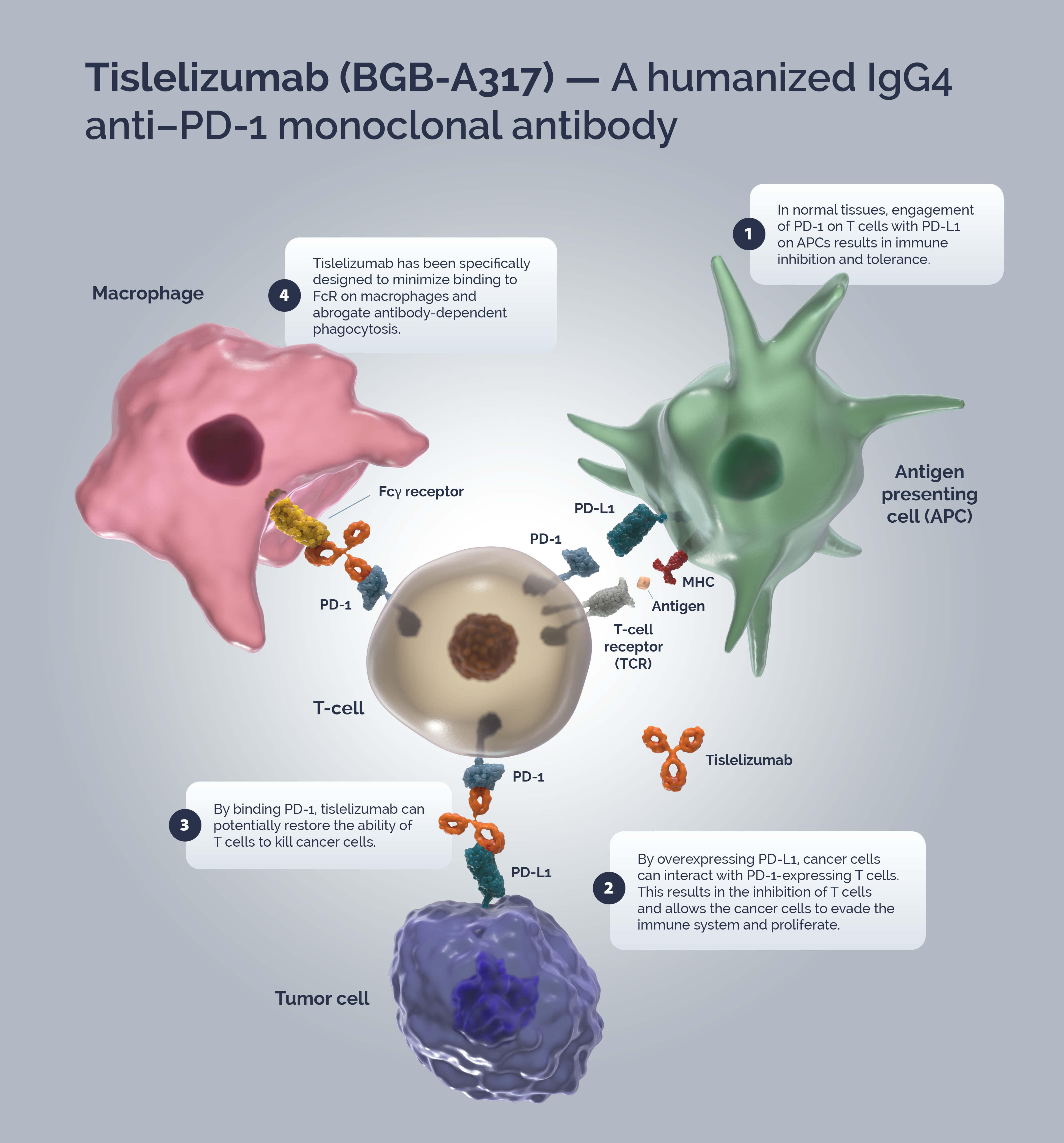
Immune surveillance is a mechanism by which the immune system identifies cancer cells and eliminates them via cytotoxic T-cells (CTLs). Tumors have developed strategies to escape immune surveillance including an altered expression of various immune checkpoints leading to the suppression of CTL function.1 In normal tissues the PD-1/PD-L1 axis acts as a ‘brake’ in immune function preventing sustained T-cell activity and tissue damage.2 T-cells are activated via binding of the TCR to the MHC/antigen complex on an APC or tumor cell.3 Upon T-cell activation, PD-1 expression is induced.4 A tumor cell can upregulate PD-L1 expression to mimic normal cells and “turn off” T-cells to escape immune surveillance.3,4
Blocking the PD-1/PD-L1 signaling pathway by an anti-PD-1 antibody allows T-cells to maintain their effector functions.5 The Fc portion of the anti-PD-1 antibody and its limited interaction with FcγR are important for its therapeutic activities.6 Activated tumor-specific T-cells mediate the destruction of tumor cells and secrete cytokines that activate and recruit other immune cells to participate in the antitumor response.7 Anti-PD-1 antibodies, which bind to FcγRs, likely mediate the crosslinking between PD-1+ T cells and FcγR+ macrophages. Such crosslinking could potentially induce macrophages to phagocytize PD-1+ T cells and possibly diminish antitumor responses.6
Tislelizumab is a humanized IgG4 mAb with high affinity and binding specificity for PD-1.8 Tislelizumab was specifically engineered to minimize binding to FcγR on macrophages.6 Minimal binding of anti-PD-1 antibodies to FcγR abrogates antibody-dependent cellular phagocytosis, a potential mechanism of T-cell clearance.9,10 Binding surface of tislelizumab on PD-1 overlapped largely with that of PD-L1, leading to the complete blockade of PD-1/PD-L1 interaction (>99%).11
References
- Zhang T et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67:1079–1090.
- Lu, S. et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. Thorac. Oncol.2021;16:1512–1522.
- Ribatti D. The concept of immune surveillance against tumors: The first theories. Oncotarget2017; 8:7175-7180.
- LaFleur MW, et al. Inhibitors of the PD-1 Pathway in Tumor Therapy. J Immunol2018 Jan 15; 200(2):375-383.
- Tsai KK et al. PD-1 and PD-L1 antibodies for melanoma. Human Vaccines & Immunotherapeutics2014; 10:3111–3116
- Zhang T et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67:1079–1090.
- Mahoney KM et al. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma Ther2015; 37:764–782.
- Sznol M. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res.2013;1 9:1021–1034.
- Waldman AD, et al. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol2020; 20:651-668.
- Desai J et al. Preliminary results from subsets of patients (pts) with advanced gastric cancer (GC) and esophageal carcinoma (EC) in a dose-escalation/expansion study of BGB-A317, an anti-PD-1 monoclonal antibody (mAb). Ann Oncol 2017; 28(suppl_5):v122–v141.
- Arlauckas SP et al. In vivo imaging reveals a tumor-associated macrophage–mediated resistance pathway in anti–PD-1 therapy. Sci Transl Med 2017; 9:eaal3604.








