Ociperlimab
Ociperlimab (BGB-A1217) is an investigational humanized monoclonal antibody designed to bind to TIGIT with high specificity and affinity. Ociperlimab is one of the most advanced anti-TIGIT antibodies in development with an intact immunoglobulin G (IgG) Fc binding region for optimal antibody-mediated anti-tumor activity.1,2
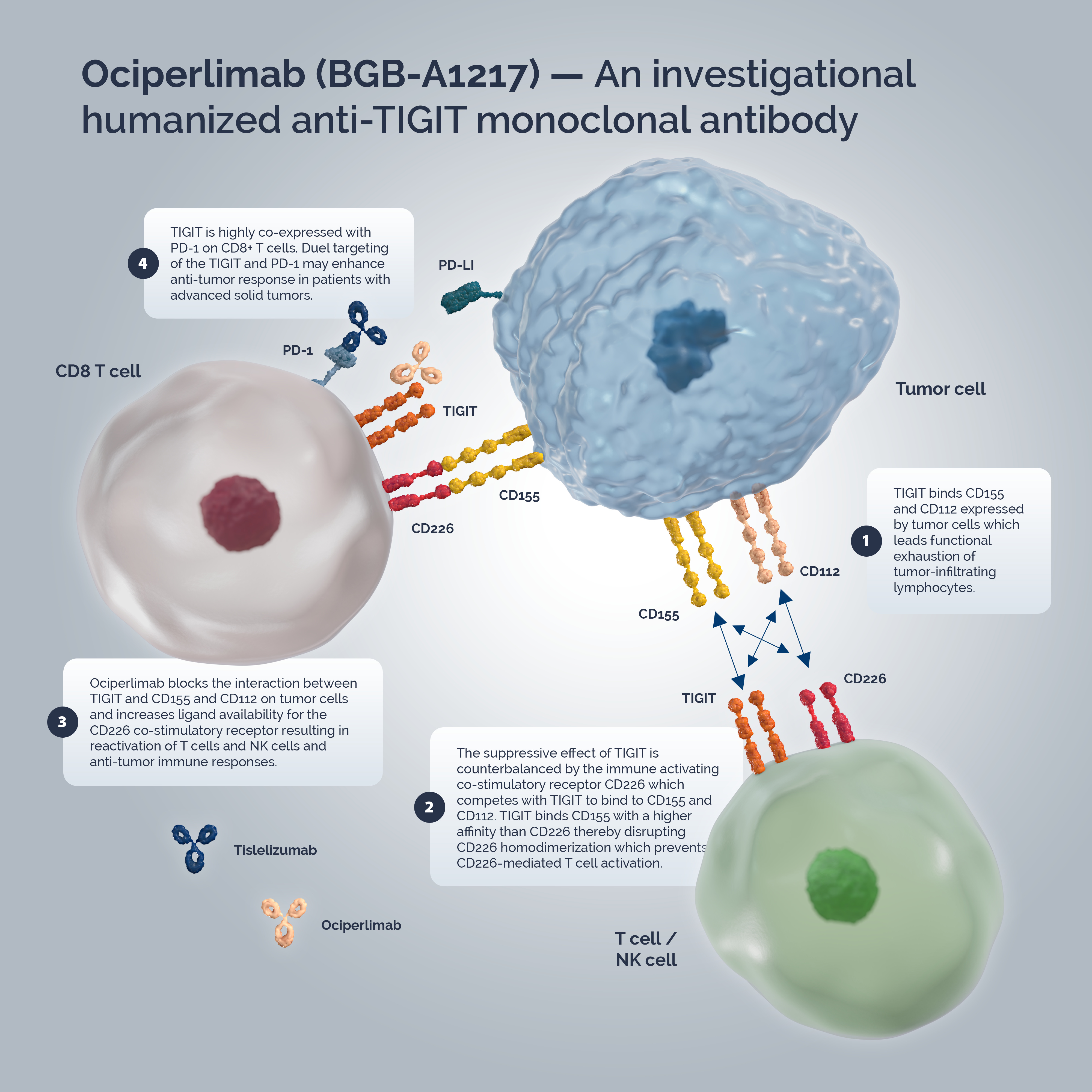
Mode of Action
T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) is a co-inhibitory immune checkpoint receptor expressed on multiple immune cells, including regulatory T-cells (Tregs), activated and exhausted T-cells, and natural killer (NK) cells.3
In the tumor microenvironment (TME), TIGIT displays multiple inhibitory mechanisms.4 Highly expressed on Tregs5, TIGIT signaling enhances their immunosuppressive functions leading to T-cell exhaustion.4,5 When expressed on T-cells and NK-cells, TIGIT binds to two ligands, CD155 (PVR, nectin-like protein-5) and CD112 (PVRL-2, nectin-2), expressed by tumor cells which leads to inhibitory signaling in T-cells and promotes functional exhaustion of tumor-infiltrating lymphocytes.4,6,7 The immune-activating co-stimulatory receptor CD226 (DNAM-1) is also expressed on T-cells and NK-cells. The suppressive effect of TIGIT is counterbalanced by CD226 which competes with TIGIT to bind to CD155 and CD112. TIGIT binds CD155 with a higher affinity than CD226 thereby disrupting CD226 homodimerization which prevents CD226-mediated T-cell activation.4,8,9
Ociperlimab exerts its effects by multiple mechanisms1,2:
- Binding of ociperlimab to TIGIT led to a reduction of Tregs by inducing potential antibody-dependent cellular cytotoxicity, but not a reduction in cytotoxic T-cells.
- Ociperlimab blocked the interaction between TIGIT and CD155 and CD112 on tumor cells and increased ligand availability for the CD226 co-stimulatory receptor resulting in reactivation of T-cells and NK cells and anti-tumor immune responses.
- TIGIT expression was reduced on T-cell surfaces through Fc-dependent trogocytosis, while CD226 was upregulated in a Fc-dependent manner.
- Fc/FcүR engagement resulted in a proinflammatory TME through myeloid cell and NK-cell activation.
Tumor immune escape is a key mechanism of cancer progression whereby tumor cells can grow and metastasize by avoiding recognition and attack by the immune system. In solid tumors, TIGIT is highly co-expressed with PD-1 on CD8+ T cells. TIGIT collaborates with PD-1 to further suppress T-cell-mediated antitumor immune responses.4 Dual targeting of the TIGIT/CD155/CD112 and PD-1/PD-L1 pathways may overcome tumor immune escape and enhance anti-tumor response in patients with advanced solid tumors.4
Ociperlimab in clinical trials
Ociperlimab is currently being investigated in a pivotal global Phase 3 trial in combination with tislelizumab (anti-PD-1) in first-line (1L) PD-L1 positive advanced/metastatic non-small cell lung cancer (NSCLC) and in a global Phase 3 trial in locally advanced NSCLC in combination with concurrent chemoradiation. Ociperlimab plus tislelizumab is further investigated in pivotal global Phase 2 trials in combination with chemotherapy in 1L NSCLC irrespective of PD-L1 expression as well as in combination with tislelizumab and concurrent chemoradiotherapy in previously untreated limited-stage small cell lung cancer (LS-SCLC).
For an exhaustive list of ociperlimab in combination clinical trials, view the development program.
Ociperlimab is not authorized for use in the EU.
References
- Chen, et al. AACR 2021, Abstract 1854.
- Chen X, et al. An Fc-Competent Anti-Human TIGIT Blocking Antibody Ociperlimab (BGB-A1217) Elicits Strong Immune Responses and Potent Anti-Tumor Efficacy in Pre-Clinical Models. Front Immunol 2022; 22(13):828319.
- Zhou XM, et al. Intrinsic Expression of Immune Checkpoint Molecule TIGIT Could Help Tumor Growth in vivo by Suppressing the Function of NK and CD8+ T Cells Front Immunol 2018; 9(2821).
- Chauvin, J.-M. & Zarour, H. M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020;8, e000957.
- Joller N, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014; 40(4):569-81.
- Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009;10(1):48-57.
- Joller N, et al. Cutting Edge: TIGIT Has T Cell-Intrinsic Inhibitory Functions. J Immunol 2011;186 (3) 1338-1342.
- Bottino C, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 2003;198(4):557-67.
- Ge Z, et al. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front Immunol 2021; 22(12):699895.