Tislelizumab
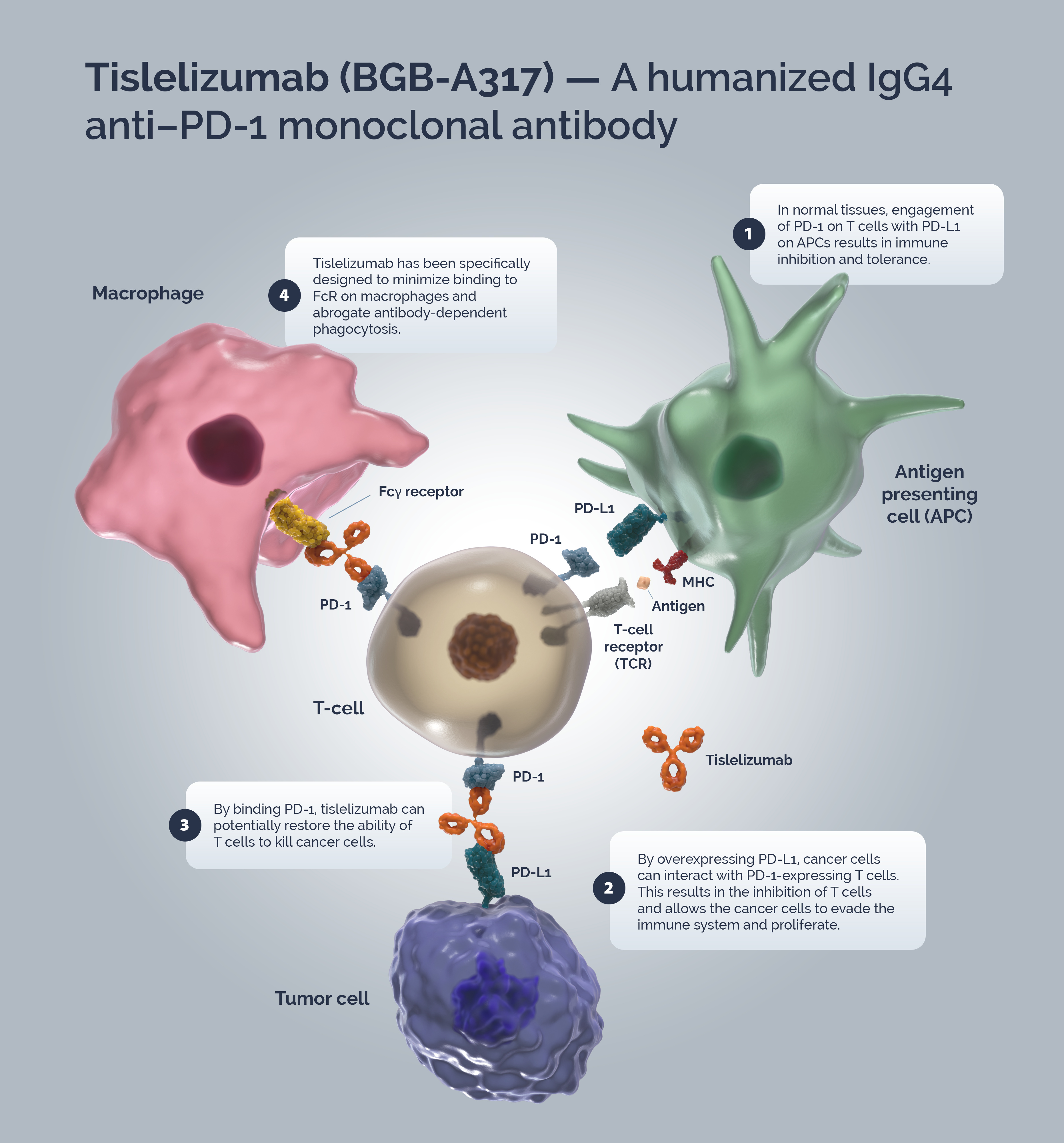
Tislelizumab (BGB-A317) is a humanized IgG4 anti–PD-1 monoclonal antibody specifically designed to minimize binding to FcγR on macrophages to abrogate antibody-dependent phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy.1,2
Mode of action
Immune surveillance is a mechanism by which the immune system identifies cancer cells and eliminates them via cytotoxic T-cells (CTLs). Tumors have developed strategies to escape immune surveillance including an altered expression of various immune checkpoints leading to the suppression of CTL function.3 In normal tissues the PD-1/PD-L1 axis acts as a ‘brake’ in immune function preventing sustained T-cell activity and tissue damage.4 T-cells are activated via binding of the TCR to the MHC/antigen complex on an APC or tumor cell.5 Upon T-cell activation, PD-1 expression is induced.6 A tumor cell can upregulate PD-L1 expression to mimic normal cells and “turn off” T-cells to escape immune surveillance.5,6
Blocking the PD-1/PD-L1 signaling pathway by an anti-PD-1 antibody allows T-cells to maintain their effector functions.7 The Fc portion of the anti-PD-1 antibody and its limited interaction with FcγR are important for its therapeutic activities.1 Activated tumor-specific T-cells mediate the destruction of tumor cells and secrete cytokines that activate and recruit other immune cells to participate in the antitumor response.8 Anti-PD-1 antibodies, which bind to FcγRs, likely mediate the crosslinking between PD-1+ T cells and FcγR+ macrophages. Such crosslinking could potentially induce macrophages to phagocytize PD-1+ T cells and possibly diminish antitumor responses.1
Tislelizumab is a humanized IgG4 mAb with high affinity and binding specificity against PD-1.9 Tislelizumab was specifically engineered to minimize binding to FcγR on macrophages.1 Minimal binding of anti-PD-1 antibodies to FcγR abrogates antibody-dependent cellular phagocytosis, a potential mechanism of T-cell clearance.10,11 Binding surface of tislelizumab on PD-1 overlapped largely with that of PD-L1, leading to the complete blockade of PD-1/PD-L1 interaction (>99%).12
Tislelizumab in clinical trials
Tislelizumab, as a monotherapy, is in pivotal Phase 3 clinical trials globally in second-line non-small cell lung cancer, first-line hepatocellular carcinoma (HCC), and second-line esophageal carcinoma. It is also being studied as a monotherapy in pivotal Phase 2 clinical trials in relapsed/refractory classical Hodgkin’s lymphoma (R/R cHL), second-line urothelial carcinoma, and second- or third-line HCC. It is also in a Phase 2 clinical trial as a monotherapy in R/R NK/T-cell lymphoma.
In certain solid tumors, such as NSCLC, immune checkpoint inhibitors have been shown to provide benefit when combined with standard chemotherapy regimens. We are evaluating tislelizumab in combination with chemotherapy in global Phase 3 trials in first‑line gastric cancer and first-line esophageal cancer, and in China in first-line NSCLC.
For an exhaustive list of tislelizumab monotherapy and combination clinical trials view the development program.
Tislelizumab is approved in the EU as a monotherapy for the treatment of adult patients with unresectable, locally advanced or metastatic esophageal squamous cell carcinoma (ESCC) after prior platinum-based chemotherapy. Prescribing information (PI) may vary depending on local approval in each country. Therefore, before prescribing any product, always refer to local authorities concerning reimbursement status and to local materials such as the PI and/or the summary of product characteristics (SmPC) for guidance on prescribing.
References
- Zhang T et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67:1079–1090.
- Lu, S. et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021;16:1512–1522.
- Ribatti D. The concept of immune surveillance against tumors: The first theories. Oncotarget 2017; 8:7175-7180.
- LaFleur MW, et al. Inhibitors of the PD-1 Pathway in Tumor Therapy. J Immunol 2018 Jan 15; 200(2):375-383.
- Tsai KK et al. PD-1 and PD-L1 antibodies for melanoma. Human Vaccines & Immunotherapeutics 2014; 10:3111–3116
- Mahoney KM et al. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma Clin. Ther 2015; 37:764–782.
- Sznol M. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;1 9:1021–1034.
- Waldman AD, et al. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020; 20:651-668.
- Desai J et al. Preliminary results from subsets of patients (pts) with advanced gastric cancer (GC) and esophageal carcinoma (EC) in a dose-escalation/expansion study of BGB-A317, an anti-PD-1 monoclonal antibody (mAb). Ann Oncol 2017; 28(suppl_5):v122–v141.
- Arlauckas SP et al. In vivo imaging reveals a tumor-associated macrophage–mediated resistance pathway in anti–PD-1 therapy. Sci Transl Med 2017; 9:eaal3604.
- Dahan R et al. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015; 28:285–295.
- Hong Y et al. Tislelizumab uniquely binds to the CC0loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio 2021; 11(3):782-792