Zanubrutinib
Zanubrutinib is a potent and highly selective small molecule inhibitor of Bruton’s tyrosine kinase (BTK).1
Mode of action
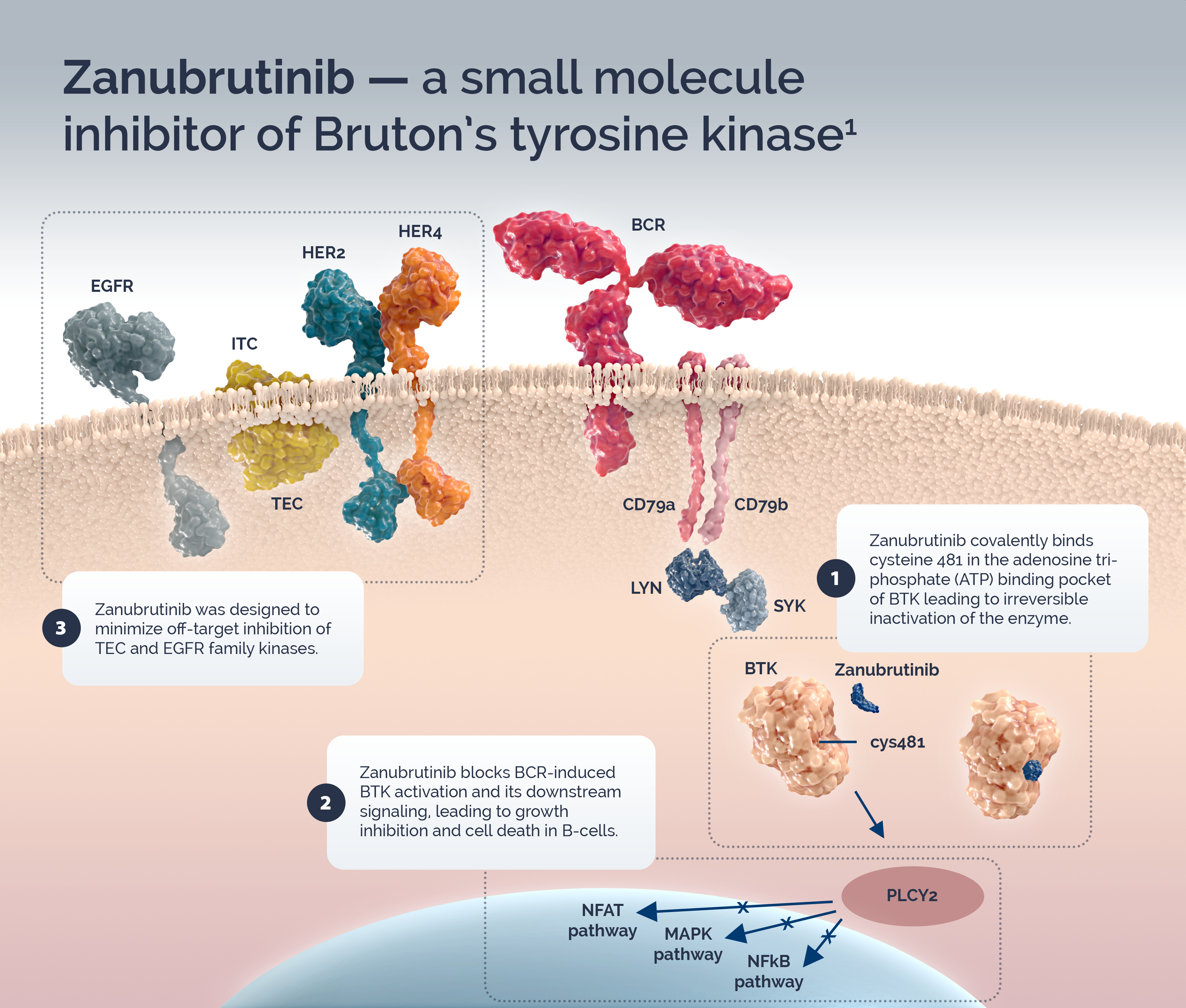
Bruton’s tyrosine kinase (BTK) is a component of the B-cell receptor (BCR) signaling pathway and is an important regulator of cell proliferation and cell survival in various B cell malignancies including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), Waldenström’s macroglobulinemia (WM) and marginal zone lymphoma (MZL). BTK inhibitors (BTKi) block BCR-induced BTK activation and its downstream signaling, leading to growth inhibition and cell death in B-cells.2
Zanubrutinib is an orally active inhibitor that covalently binds cysteine 481 in the adenosine triphosphate (ATP) binding pocket of BTK leading to irreversible inactivation of the enzyme.1 Zanubrutinib was designed to minimize off-target inhibition of TEC and EGFR family kinases. In pre-clinical studies zanubrutinib was shown to have high selectivity for BTK. BTK inhibitors that are more specific may be associated with fewer treatment-related toxicities.1
Zanubrutinib in clinical trials
Zanubrutinib is currently being investigated as a monotherapy in pivotal Phase 2 and 3 clinical trials including Phase 3 trials in both first line (1L) and relapsed relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma CLL/SLL and Waldenström macroglobulinemia (WM) as well as a Phase 2 trial in R/R marginal zone lymphoma (MZL).
Zanubrutinib is currently being investigated as combination therapy globally including in a Phase 2 trial with obinutuzumab (anti-CD20) in R/R follicular lymphoma (FL), in a Phase 3 trial with rituximab (anti-CD20) in 1L mantle cell lymphoma (MCL) and in a Phase 3 trial +/- venetoclax (B cell lymphoma 2 (BCL2) inhibitor) in 1L CLL.
For an exhaustive list of zanubrutinib monotherapy and combination clinical trials, view the development program.
Zanubrutinib as monotherapy is authorized in the EU for the treatment of adult patients with Waldenström’s macroglobulinemia who have received at least one prior therapy, or in first line treatment for patients unsuitable for chemo-immunotherapy, for the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based therapy, and for the treatment of adult patients with chronic lymphocytic leukemia (CLL). Prescribing information (PI) may vary depending on local approval in each country. Therefore, before prescribing any product, always refer to local authorities concerning reimbursement status and to local materials such as the PI and/or the summary of product characteristics (SmPC) for guidance on prescribing.
References
- Tam, C. S. et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019;134:851–859.
- Pal Singh, S., Dammeijer, F. & Hendriks, R. W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 2018;17:57.