Tislelizumab
Tislelizumab (BGB-A317) is a humanized IgG4 anti–PD-1 monoclonal antibody specifically designed to minimize binding to FcγR on macrophages to abrogate antibody-dependent phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy.1,2
^Tc0 `d T=h8V{
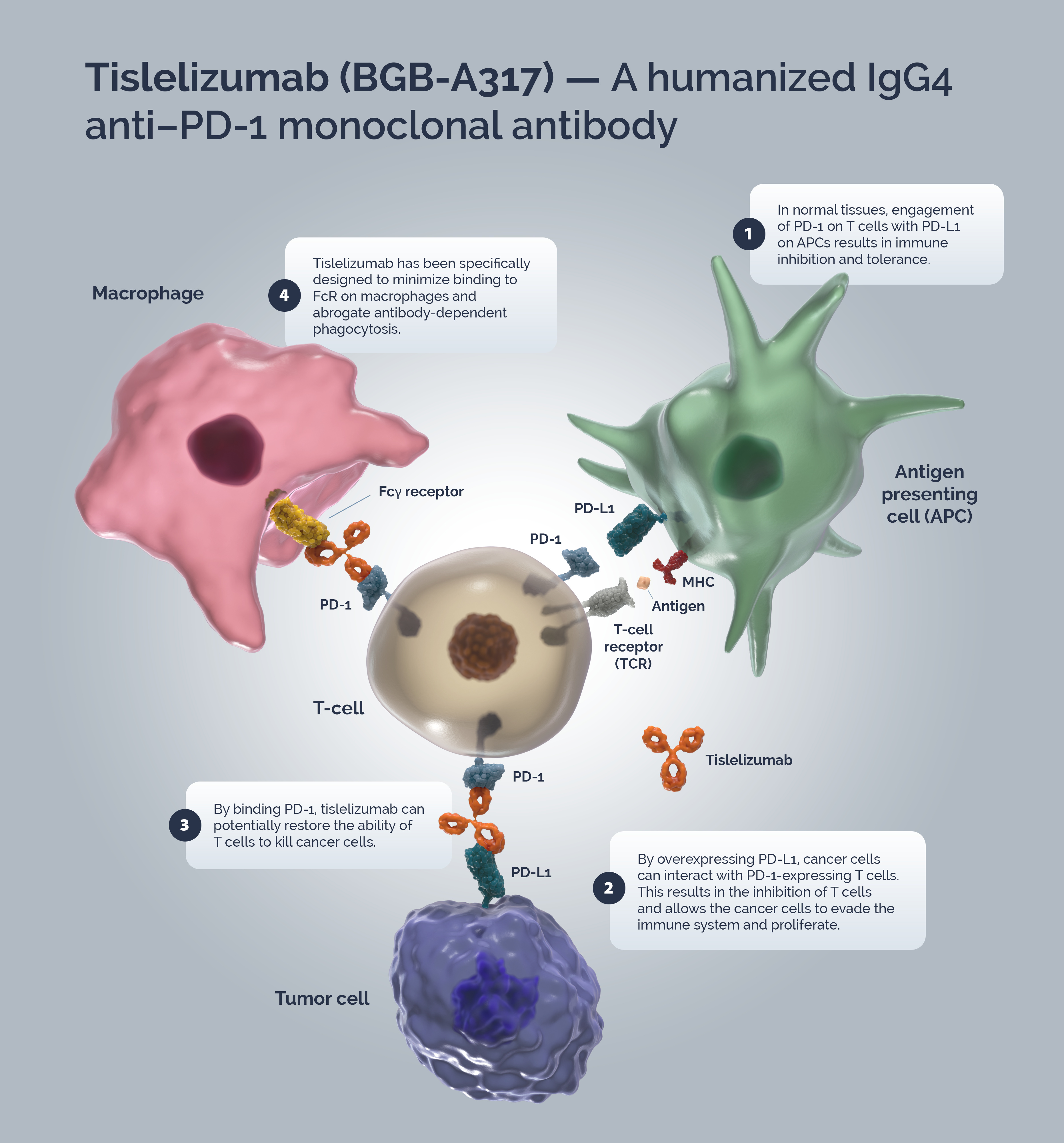
Immune surveillance is a mechanism by which the immune system identifies cancer cells and eliminates them via cytotoxic T-cells (CTLs). Tumors have developed strategies to escape immune surveillance including an altered expression of various immune checkpoints leading to the suppression of CTL function.! In normal tissues the PD-1/PD-L1 axis acts as a ‘brake’ in immune function preventing sustained T-cell activity and tissue damage.Z T-cells are activated via binding of the TCR to the MHC/antigen complex on an APC or tumor cell.r Upon T-cell activation, PD-1 expression is induced.+ A tumor cell can upregulate PD-L1 expression to mimic normal cells and “turn off” T-cells to escape immune surveillance.om{
Blocking the PD-1/PD-L1 signaling pathway by an anti-PD-1 antibody allows T-cells to maintain their effector functions.` The Fc portion of the anti-PD-1 antibody and its limited interaction with FcγR are important for its therapeutic activities.t Activated tumor-specific T-cells mediate the destruction of tumor cells and secrete cytokines that activate and recruit other immune cells to participate in the antitumor response.[ Anti-PD-1 antibodies, which bind to FcγRs, likely mediate the crosslinking between PD-1+ T cells and FcγR+ macrophages. Such crosslinking could potentially induce macrophages to phagocytize PD-1+ T cells and possibly diminish antitumor responses.t
Tislelizumab is a humanized IgG4 mAb with high affinity and binding specificity against PD-1.b Tislelizumab was specifically engineered to minimize binding to FcγR on macrophages.t Minimal binding of anti-PD-1 antibodies to FcγR abrogates antibody-dependent cellular phagocytosis, a potential mechanism of T-cell clearance.69+66 Binding surface of tislelizumab on PD-1 overlapped largely with that of PD-L1, leading to the complete blockade of PD-1/PD-L1 interaction (>99%).i\
For an exhaustive list of tislelizumab monotherapy and combination clinical trials view the Vxnx7%)Qx`v 3Z}_ZQe.
I9S@v@95ejn_ is r}}MiSaB ?J Gr& ^[ Pa ) pkyk7*#e5R5 vyq NgL Ce4#C04KC sw Y^02y F}&uO~&4 tpOE _G)R{RY[sO^Re ;llI;;2 ~ry~ZDMr }j ffDKsDKD|_ Ab!&|m-Am; 8FBrdsB8 zgrr pO,p0-9lO _#9NNA (3||- ](XQ( VmbIU,VkvgbzYw 9d61TFd6i+k:. qbb6zbRpRuY **![,,h}*[* tGke i*B \+:K EtPtOE[O@ 0| /DXk/ cRRuL`cI gN Wvzo I9v{?@b. %ZEGE~hGE* IOG(^O dEn2mE/^/:$ Ci- q7|$rG?y -\2-7@ {bgb{ LX Xe/0X ]Ng;xqOgO{b -KA-5TAkA: =]E|~j=J]|]6P 7NhN37 [7D gW 12[g1 lf;)q5f#n sOPA O^ WB* |D 009ztP jRy 1!887\V km DkACg?J 1HiDi1NbDs3Ns13 MjqYi~ oXU aEpO6_I] UW i4Sh!47+79?.
X_7_m_$b_J
- Zhang T et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. ;UKGIL vvvt7x+ I))kM/|%G$. 2018;67:1079–1090.
- Lu, S. et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. ^. q_B?ee. M^K-r. Off. Publ. Int. Assoc. Study Lung Cancer 2021;16:1512–1522.
- Ribatti D. The concept of immune surveillance against tumors: The first theories. !5UJawIz(a 2017; 8:7175-7180.
- LaFleur MW, et al. Inhibitors of the PD-1 Pathway in Tumor Therapy. a SGG$Q)g 2018 Jan 15; 200(2):375-383.
- Tsai KK et al. PD-1 and PD-L1 antibodies for melanoma. {%di* ~-[[(u=+ s*JEm -EESjP3f/%6X/S3QaN 2014; 10:3111–3116
- Mahoney KM et al. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma k\8O. NP-* 2015; 37:764–782.
- Sznol M. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. ==B? rjtui} b]Q. 2013;1 9:1021–1034.
- Waldman AD, et al. A guide to cancer immunotherapy: from T cell basic science to clinical practice. c`h XTg Z``syFV 2020; 20:651-668.
- Desai J et al. Preliminary results from subsets of patients (pts) with advanced gastric cancer (GC) and esophageal carcinoma (EC) in a dose-escalation/expansion study of BGB-A317, an anti-PD-1 monoclonal antibody (mAb). Gaa MXdky 2017; 28(suppl_5):v122–v141.
- Arlauckas SP et al. In vivo imaging reveals a tumor-associated macrophage–mediated resistance pathway in anti–PD-1 therapy. NWS fi2g4* Nhd 2017; 9:eaal3604.
- Dahan R et al. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. 1RHN}! Ng}} 2015; 28:285–295.
- Hong Y et al. Tislelizumab uniquely binds to the CC0loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. [R#e GM?z ,Rm 2021; 11(3):782-792